Understanding soil pH Part I
Soil pH is a standard characteristic measured by a soil test, but what does the number mean and how does pH affect plant growth? Here’s a straight forward look at understanding pH that will help you get the maximum benefit from this measurement.
One of my college professors stated the only sentence that can start with a lower case letter is one starting with the word pH. pH, however, is more important to crop production than just a grammatical oddity. Soil pH affects nutrient availability, heavy metal mobility, soil microbe activity, pesticide effectiveness and other characteristics critical to production success.
pH is a measurement of the power of hydrogen (hence “pH”) or H+ ions. pH is measured on a 0 (extremely acid – pure Hydrochloric acid) to 14 (extremely alkaline – pure sodium hydroxide) scale, with 7 (the concentration of H+ ions at room temperature in pure water) being neutral. Both extremes are damaging to plants, which generally prefer values between 6 and 7.
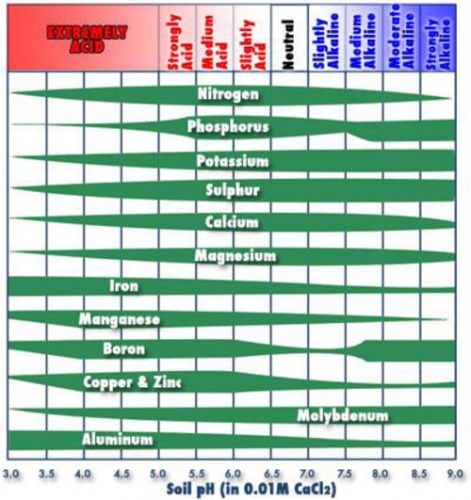
One of the biggest reasons pH is important is because it affects nutrient availability. Most nutrients are available in good quantities around 6.5 (Figure 1). In Figure 1, the wider the bar the more available the nutrient, however, the skinny portions don’t mean the element is not there, it means it is not available to the plant. Some organic soils have pH of 7.5 or higher, which means plants potentially will show copper, zinc, iron, boron and manganese deficiencies. It is too difficult to change pH of most organic soils, so the alternative is to apply these nutrients as foliar sprays during the growing season. Phosphorous is limiting in low pH soils, especially early in the season when soil is still wet and cool. That is why many blueberry plantings have purple, phosphorous deficient leaves that will eventually turn green when temperature warms.
pH is expressed on a base 10 logarithmic (log) scale, which means there is a ten-fold difference between numbers. That means 4 is 10 times more acidic than 5, 100 times more acidic than 6 and 1,000 times more than 7. This causes more confusion; how can a lower number be more acidic? This is difficult to explain, but I’ll try. The formula is: pH = -log[H+]. So, pH is a negative (-) log number which is expressed as a fraction with 1 as the numerator (top number) and the pH value as the denominator (bottom number). When placed as the denominator, the smaller number yields a larger amount of the whole (1/3 versus 1/8). That is probably not an acceptable explanation if I was a chemist, but it gets the idea across.
The main elements affecting soil pH are calcium, magnesium and potassium and on their own these elements keep pH on the alkaline side. Soil pH is related to how well the soil hangs onto these elements. In nature, sand typically has a lower pH than clay since water (rain) moves through sand faster than clay. Michigan has a fair amount of coral-based sands that are high in calcium, so newly exposed sand can be quite high in pH. Most non-amended, well-drained sand soils in Michigan will generally stabilize at a pH around 5.0 to 5.5, a value too low for most crops except blueberries and potatoes. Most commercial fertilizer applications also lower pH (except calcium nitrate and gypsum). When pH drops low enough, lime will be recommended to bring it back up. This is a chemical process and the reaction depends on how well lime is dispersed through the soil and soil temperature. The amount of lime needed depends on soil type. Because there are fewer binding sites, sand is easier to change than clay, silt or organic soils. It is not possible, or economical, to change some organic soils because there are too many sites to neutralize.
Occasionally, pH needs to be lowered. If it only needs to be lowered slightly, it can be done through standard fertilizing practices using an acidic fertilizer such as ammonium sulfate. If more significant changes are needed, you will have to add sulfur. Sulfur rate depends on soil type – don’t guess, get a soil test done. Lowering pH with sulfur is a biological process. Soil borne bacteria takes sulfur, combines it with oxygen and water to make sulfuric acid. The rate this occurs depends on the amount of water and oxygen in the soil and temperature. If sulfur is applied in the fall, little change occurs until the next year when the soil warms. Aluminum sulfate is often recommended to lower pH, but I caution against using it since the aluminum portion can become toxic and becomes more available at a lower pH.
I will continue my pH discussion in my next article, “Understanding soil pH Part II.”



 Print
Print Email
Email

