Diagnosing plant problems – don’t forget about pH and soluble salt content
Editor’s note: This article is from the archives of the MSU Crop Advisory Team Alerts. Check the label of any pesticide referenced to ensure your use is included.
Our plant diagnostic laboratory routinely inspects plants to determine the specific reason(s) of poor or improper growth. As diagnosticians, we logically and rationally rule out potential causal agents and hopefully come to a confident, definitive conclusion as to the reason of plant decline. Is the problem associated with plant pathogens, insect pests, parasitic nematodes, pesticides, inadequate nutrition, environmental constrains, poor site location or a host of other factors?
Two of the first things we attempt to rule out on every sample are problems related to plant diseases and insect pests. Once biotic factors are excluded, we then consider the many abiotic, non-living factors that could be at play. To help in this endeavor, we generally test the growth media for pH and soluble salt content. These two readings are simple, quick and can be very revealing as to the reason for poor growth.

Photo 1. One healthy Dianthus seedling
(left) versus two injured seedlings (right).
Media pH
Most plants have an optimal pH range in which they perform best. Some plants may prefer slightly acidic media while other plants can tolerate a much lower or higher pH. The pH of the media influences the uptake and availability of all nutrients. Growing greenhouse plants in media with a pH lower than the optimal range can result in poor uptake of macronutrients and increased uptake of some micronutrients (e.g. iron toxicity). On the flipside, micronutrient deficiencies generally occur on plants growing in media with a pH higher than the optimal range (e.g., iron or manganese deficiencies).
Soluble salt content
The soluble salt content of the media and irrigation water should be tested frequently throughout the growing process. All soluble nutrients, such as nitrate, ammonium, potassium, calcium, magnesium, chloride, sodium and sulfate, contribute to the soluble salt content of the media. A soluble salts test will alert growers if the salt level starts to creep upward and can also provide an indication of the nutrient status available for plant uptake.
Greenhouse plants vary in their tolerance to salt levels and this tolerance is often dependent upon the growth stage of the plant. Excessive soluble salts can result in reduced growth and loss of vigor, root death, chlorosis and necrosis of leaves, wilting and marginal leaf burn. Excessive soluble salts are generally a result of too much fertilizer present in the soil solution in relation to the plant’s needs. High salts may occur from miscalculation of fertilizer dilutions, poorly calibrated or malfunctioning fertilizer injectors or over-application of fertilizers. Other causes of excessive salt buildup may be inadequate leaching and poor drainage. Excessive soluble salts may be reduced in the media by leaching several times with clear water.
Total soluble salt content is determined with a solu-bridge conductivity meter and is expressed as millisiemen (mS) or millimho (mmho). Millisiemen is the preferred unit for expressing soluble salt measurements. Regardless of the unit, the value is the same. Growers often determine soluble salt content by using one part media to two parts distilled water, allowing the solution to sit idle for up to one hour before taking a reading. Many testing labs determine soluble salt content on the saturation extract. Guidelines for interpreting soluble salt levels in growth media are listed in Table 1.
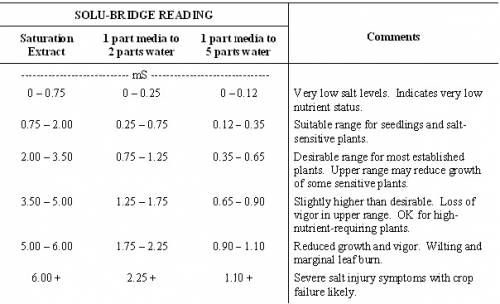
Table 1. Guidelines for interpreting soluble salt levels in growth media.
Recent greenhouse sample
We recently received a Dianthus plug sheet with stunted plants with very poor root development near the middle of the sheet compared with very healthy and well-rooted plugs along the edges (view pictures). There were no signs of plant pathogens or insect-related problems. Because there appeared to be a pattern of injury, both pH and soluble salt content was recorded from two areas of the tray. One sample was taken along the edge of the sheet – the other from the center of the sheet. While pH did not differ across the plug sheet, the plugs near the middle of the sheet contained a soluble salt content of 2.1 mS/cm compared with 0.8 mS/cm along the edges (1 part media: 2 parts water). This constituted approximately 2.5 times the soluble salts content and severe salt injury on Dianthus plugs would be expected at this level.

Photo 2. Pattern of injury in a Dianthus plug
sheet.
The jury is still out on this sample. Are the salt levels high in the middle of the Dianthus plug sheet because some other abiotic factor inhibited growth and therefore water and nutrient uptake? Or was salt toxicity an issue from the start? We are leaning towards the later – a salt toxicity problem soon after seeding which delayed germination, emergence and plant development. However, we cannot definitively determine which problem occurred first.



 Print
Print Email
Email

