Nematode analysis
Detecting and avoiding nematode problems
Plant parasitic nematodes are microscopic round worms that live in soil and feed on plant roots or foliage. Nematode feeding can result in diseased plants with symptoms such as stunting, yellowing, wilting, yield reduction, root galling and the formation of root lesions. Although damage from plant parasitic nematodes costs Michigan growers millions of dollars annually, many of these losses are never correctly diagnosed. Here we provide instructions for the methods necessary to avoid or diagnose nematode problems.
A laboratory analysis of soil, root, or shoot system tissue is necessary for diagnosis or long-term avoidance of plant-parasitic nematode problems.
There are two primary objectives when sampling for nematodes: problem avoidance and problem diagnosis. Sampling methodologies can differ depending on your sampling objective.
Avoiding nematode problems
Generally, soil from Michigan agricultural sites should be analyzed for nematodes every 3-5 years. The sample results are used to make decisions for managing and avoiding nematode problems.
Plants/Crops differ in their susceptibilities to nematodes. When trying to avoid nematode problems, this fact is important to keep in mind. In addition, the amount of disease (virulence) caused by nematodes differs significantly depending on the kinds (genera and species) of nematodes present. For these reasons, it is imperative samples are collected and analyzed if you best want to avoid and manage plant-parasitic nematodes.
There are more tactics available to manage nematodes before crops go into the ground than after planting. For some crops, there are post-plant nematicides available, but these are generally just available for perennial plants (although there are some exceptions). In addition, many nematicides (substances that kill nematodes) work on contact with nematodes in the soil. If a product has no systemic activity, it will provide no control of nematodes already established within roots or foliage. For these and other reasons, avoidance of plant-parasitic nematodes should be the goal of all plant producers.
When to sample
Generally, soil and root samples can be taken, submitted, and reliably processed whenever the soil is not frozen. To avoid nematodes in annual cropping systems, it is necessary to collect samples prior to planting. In Michigan, sampling from September 1st to December 1st is recommended especially if soil fumigation is an option. In perennial cropping systems, sampling once every 3 to 5 years is advised.
When submitting samples to avoid plant-parasitic nematode problems, consider the following recommendations:
- Crops that should always be sampled before establishment:
- All fruit plantings (small, pome and stone)
- Carrots, celery and parsnips
- Herbaceous perennials/nursery stock
- Hop yards
- Potatoes (nematodes and Verticillium dahliae)
- Soybeans
- Sugar beets
Justification: Dagger, needle and stubby-root nematodes can vector plant viruses. There are no effective pesticides against these viruses. Be sure to sample tree fruit planting sites and future hop yards for nematodes prior to establishment. Carrots, celery and parsnips are extremely susceptible to root-knot nematodes. In carrots and parsnips, qualitative losses due to malformed roots, can be significant. Since herbaceous perennials/nursery stock will ultimately be disseminated to customers, the spread of nematodes can be reduced if avoidance tactics are utilized during initial establishment. Most potato varieties are susceptible to potato early-die (PED). Since PED appears to be an interaction between lesion nematodes and the fungus, Verticillium dahliae, collection of samples is necessary to assay for both pathogens and assess risk. There is plant resistance/tolerance available in soybean and sugar beets against soybean cyst and sugar beet cyst nematodes, respectively. In soybean, if growers plant an SCN-susceptible soybean variety in a field where they should have used a resistant one, >50% yield loss is a strong certainty.
- Crops that should be sampled soon after establishment:
- All fruit prior to bearing
- Herbaceous perennials/nursery stock
Justification: In fruit, in particular, there is a nematicidal option(s) for use prior to plants bearing fruit. This window of opportunity also allows a fruit producer to diagnose any problems associated with the bushes, trees or vines he/she purchased. Some of the most problematic nematodes feed within root-tissue therefore, they are easily and inconspicuously transported in plant material.
- Crops/Plants that should be sampled every 3-5 years:
- All fruit plantings, except strawberry
- Forage legume fields
- Golf greens and tees
- Row-crop fields
- Vegetable fields
Justification: Most of the plants listed here do not usually suffer significant yield losses due to plant-parasitic nematodes or there are some post-plant control options available. The goal of all plant producers should be to keep nematode numbers below damage threshold levels and monitor them, so they know when they approach their action thresholds. Often, the amount of disease caused by most parasites/pathogens is a function of the initial parasite burden (in agriculture, this is the "at-plant population density"), so nematode populations should be at least occasionally monitored in all habitats where plants are grown for profit. It is rare to find fields where plant-parasitic nematodes are absent.
- Crops that should be sampled at harvest:
- Garlic
- Herbaceous perennials/nursery stock
- Sugar beets
Justification: Garlic bulbs can be consumed or used for seed. Bulbs with bloat nematodes should never be used as seed. Sample garlic at harvest for the presence of bloat nematodes. Herbaceous perennials and nursery stock can be treated after harvest for nematodes. Sugar beet cyst nematode population densities are highest after sugar beets are grown. Harvest is a good time to sample in this crop so the information can be used for decision making prior to the next beet crop.
How to sample

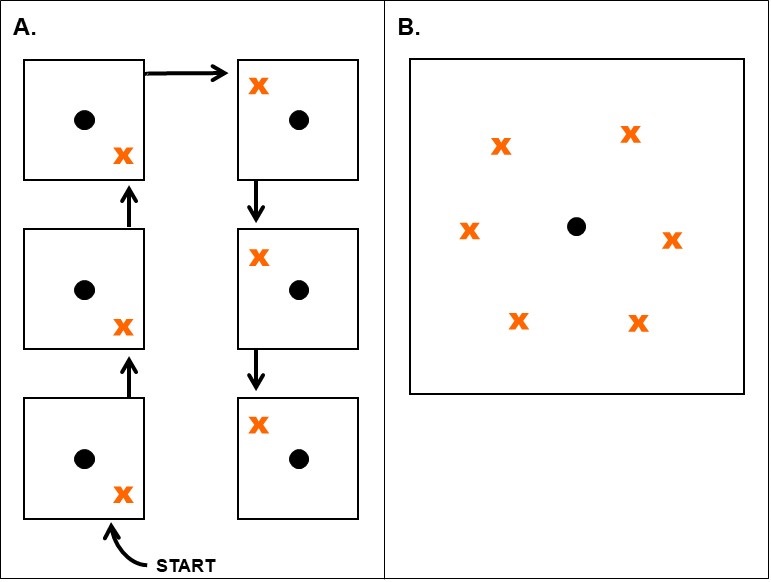
Sampling instrument: Take samples with a solid sampling tube, trowel, or narrow-bladed shovel at a 2- to 12-inch depth. Include as many feeder roots as possible (see Figure 1). Feeder roots or shoot tissue must always be included for samples submitted for recovering endoparasitic nematodes.
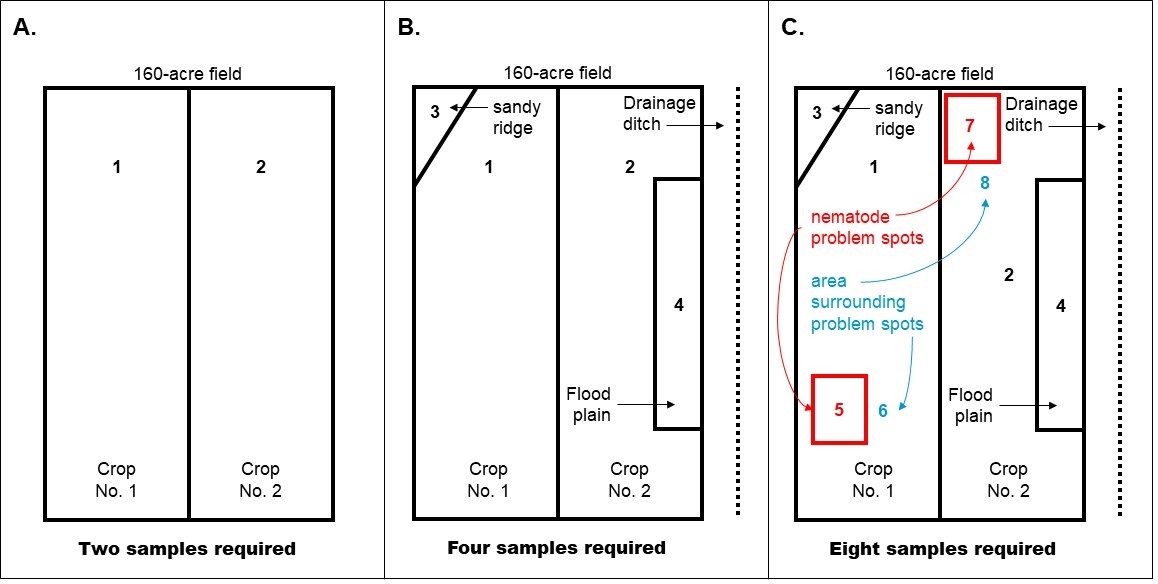
Sample size: Each sample should consist of a pint to a quart of soil taken from a larger sample composed of 10 or more subsamples (see Figure 2). The number of samples needed depends on the size, history, and uniform soil texture of the area being investigated (see Figure 3):
- Small area (less than 5,000 sq. ft.), take at least 5-10 subsamples (soil cores or borings).
- Medium area (5,000 sq. ft. to 1 acre), take at least 10-25 subsamples.
- Large area (1 to 80 acres), take at least 25-50 subsamples. In Michigan, no one sample should represent more than 80 acres, and each sample should be from an area of uniform soil texture.
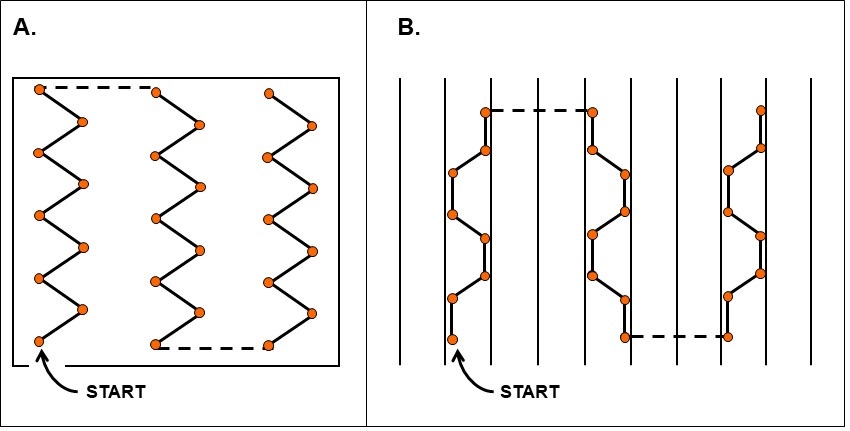
The sampling pattern depends on the commodity and field history (recommended sampling schemes are illustrated in Figures 4 and 5).
Mix subsamples in a clean pail (see Figure 2) or a plastic bag and submit one pint to a quart for nematode analysis.
Subsamples from problem area: Plant-parasitic nematodes feed only on living tissues and are rarely found in dead root samples. Therefore, take samples from the margin of the problem areas where the plants are still living.
Diagnosing nematode problems
Sampling for problem diagnosis usually occurs during the growing season. When plants exhibit symptoms such as stunting, yellowing, wilting, early-die, yield reduction, root galling, root-lesions or plant mortality that cannot be attributed to other causes, take samples of appropriate soil, root, or shoot system, and submit them for nematode analysis.
Identify spots in fields where symptoms of undetermined causes are evident and collect soil and roots from the margins of those areas. The margins of diseased areas are where nematode numbers will typically be highest. Collection of these samples can/should occur from 30 days after planting until harvest, however, there are optimal times of the season to sample for certain nematodes. For comparison purposes, it is often beneficial to sample healthy plants nearby to diseased ones and submit the samples in separate containers or bags.
For diagnosis of the pine wilt disease and other foliar diseases of ornamentals, shoot system tissue is required.
- For pine wilt, a sample submission should consist of branches of at least 2 inches in diameter and 6 inches long.
- For foliar diseases of ornamentals, entire plants or several terminal shoots showing symptoms should be submitted for testing.
Sampling container: Place samples in resealable, quart-size plastic bags as soon as possible. Nematodes will be killed if the sample is allowed to dry, and it is important that nematodes are living when the sample arrives at the laboratory.
Sampling storage: Soil and root samples should be regarded as perishable. Handle accordingly, and process as quickly as possible. Ideally, they should be stored at 50-58 F (10-15 C). Do not expose them to direct sunlight or store them in hot areas, such as the trunk of your car. Temperatures greater than 100 F (40 C) will kill nematodes.
How to submit samples
Samples in plastic bags for nematode analysis should be shipped or taken directly to:
MSU Plant & Pest Diagnostics
578 Wilson Road, CIPS 107
East Lansing, MI 48824
Every sample submission must be accompanied by a completed Nematode Analysis Form. The information requested on the form is essential for diagnosing nematode problems and proper recommendations for nematode population management.
Results and recommendations
It generally takes two weeks from the time a sample is taken until the results are returned to the grower. The results may be returned through the local extension agent, a private consultant, or directly to the grower.
The types and numbers of nematodes will be recorded on the report along with an indication of whether or not nematodes are a problem. If nematodes appear to be a problem, you will be provided with a control recommendation.
Post-treatment samples
One way to analyze the success of nematode population management is to submit a post-treatment sample for nematode analysis. These samples should be taken four to six weeks after treatment.
Testing fees
Please note that fees for out-of-state samples are double.
|
Description |
Fee per sample |
|
Basic nematode analysis |
$25.00 |
|
Foliar nematode analysis |
$25.00 |
|
Total nematode community analysis |
$50.00 |
|
Full SCN type testing |
$75.00 |
|
Mini SCN type testing |
$40.00 |
|
Verticillium wilt analysis (potato soil & stems): |
|
|
$25.00 |
|
|



 Print
Print Email
Email