Christmas trees 101: Nutrient management
Proper soil nutrient management is a win-win scenario that improves grower efficiency and protects the environment.
In our previous articles in the Christmas trees 101 series, soil test to optimize production and site selection and modifications, we discussed the importance of soil sampling and determining suitable Christmas tree planting locations. In this article, we discuss nutrient management recommendations based on the results of our example soil test and when best to make adjustments indicated by the test. Nutrient management is critical in the establishment, growth and profitability for Christmas tree production, however, over-application of fertilizer can result in wasted fertilizer expense, tree injury and environmental pollution.
Adjusting soil pH
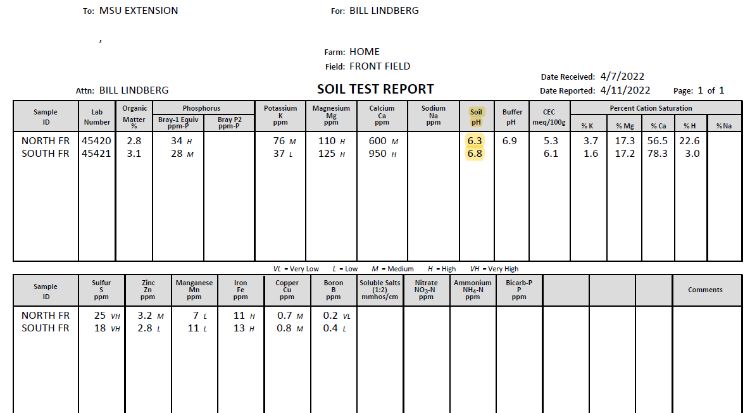
One of the most important aspects of nutrient management is maintaining a suitable soil pH. As soil pH changes, the availability of essential nutrients is altered. This impacts the ability of plants to absorb nutrients that are present in the soil. The optimal soil pH differs based on Christmas tree species (Table 1). For our example, we’ll assume we’re growing Fraser fir, which has an optimal pH range of 5.5 to 6.0. In our soil sample results (Photo 1), both samples are too basic at 6.3 and 6.8, respectively. To address this, Michigan State University Extension (MSU Extension) recommends applying elemental sulfur. Elemental sulfur lowers the pH due to the activity of bacteria in the soil. This process may take six months to a year to take full effect.
|
Table 1. Optimal soil pH ranges for Christmas tree species. |
|
|
Species |
Optimal pH range |
|
Balsam Fir |
5.5-6.0 |
|
Douglas Fir |
6.0-7.0 |
|
Fraser Fir |
5.5-6.0 |
|
Concolor Fir |
6.0-6.5 |
|
Cannaan Fir |
5.5-6.5 |
|
White Pine |
5.0-6.0 |
|
White Spruce |
5.0-6.0 |
|
Norway Spruce |
6.0-6.5 |
|
Blue Spruce |
6.0-7.0 |
|
Scotch Pine |
5.0-6.0 |
To determine the rate of application, look for the approximate starting and ending soil pH levels and the cation exchange capacity (CEC) of the soil (Table 2). The higher the CEC, the greater the buffering capacity of the soil and the more sulfur needed to lower the pH. Also, it is important to note that on heavy soil sites with a CEC of greater than 15, the amount of elemental sulfur needed may not be economically or realistically feasible to significantly lower the pH for Christmas tree production.
|
Table 2. Amount of elemental sulfur needed to lower soil pH. |
|||||
|
Initial |
Target |
Soil Cation Exchange Capacity (CEC) |
|||
|
1 |
5 |
10 |
15* |
||
|
6.0 |
5.5 |
109 |
218 |
272 |
327 |
|
6.5 |
5.5 |
218 |
436 |
545 |
654 |
|
7.0 |
5.5 |
327 |
654 |
818 |
981 |
|
7.5 |
5.5 |
436 |
872 |
1090 |
1308 |
|
6.5 |
6.0 |
80 |
160 |
200 |
240 |
|
7.0 |
6.0 |
189 |
377 |
472 |
566 |
|
7.5 |
6.0 |
343 |
686 |
870 |
1054 |
|
7.0 |
6.5 |
50 |
100 |
125 |
150 |
|
7.5 |
6.5 |
250 |
500 |
650 |
800 |
|
*Soils with CEC > 10 are resistant to change in soil pH and making an appreciable impact may be difficult |
|||||
For our example, adjusting the soil pH from 6.8 to 6.0 with a CEC = 6.1 will require about 400 pounds of elemental sulfur per acre, while shifting from 6.3 to 6.0 with a CEC = 5.3 will require about 160 pounds of elemental sulfur per acre. Most elemental sulfur products come rated as either 85% or 90% active ingredient (a.i.). For example, at 90% a.i., we would need to add 444 pounds of product (440 ÷ 0.900 to achieve 400 pounds of elemental sulfur). Applications prior to planting can be tilled into the soil, while applications in rotation can be broadcast applied.
Plant nutrient elements are grouped as macronutrients (those needed in relatively large amounts) and micronutrients (those needed in small amounts and sometimes referred to as trace elements).
Macronutrients
Nitrogen is the element typically needed in largest amounts for Christmas tree production. Nitrogen changes forms rapidly in the soil, so is not included in most soil test reports. MSU Extension recommends adjusting N fertilization to match the species and plantation age/size and use foliar testing to fine tune applications. Fertilizer application of all other elements can be based on a soil test report.
Nutrients that can benefit when applied and incorporated prior to planting include phosphorus and potassium. These nutrients move slowly through the soil profile and when incorporated will be present throughout the soil profile. Phosphorus is a potential environmental contaminant as excessive applications can contribute to eutrophication of surface water. Therefore, applications should only be made if testing indicates the soil is deficient.
To determine the rates of application, review the soil sample results and fertility recommendations (Photo 2). In our example, both sites would benefit from about 25 pounds of P2O5 (the chemical form of phosphorus in the soil). Check with your supply company on what phosphorus containing products they have available. Mono-ammonium phosphate (MAP) (11-52-0) is one of the most common products. To apply 25 pounds of P2O5, take desire rate of 25 and divide by 0.52, resulting in about 50 pounds of the MAP product per acre.
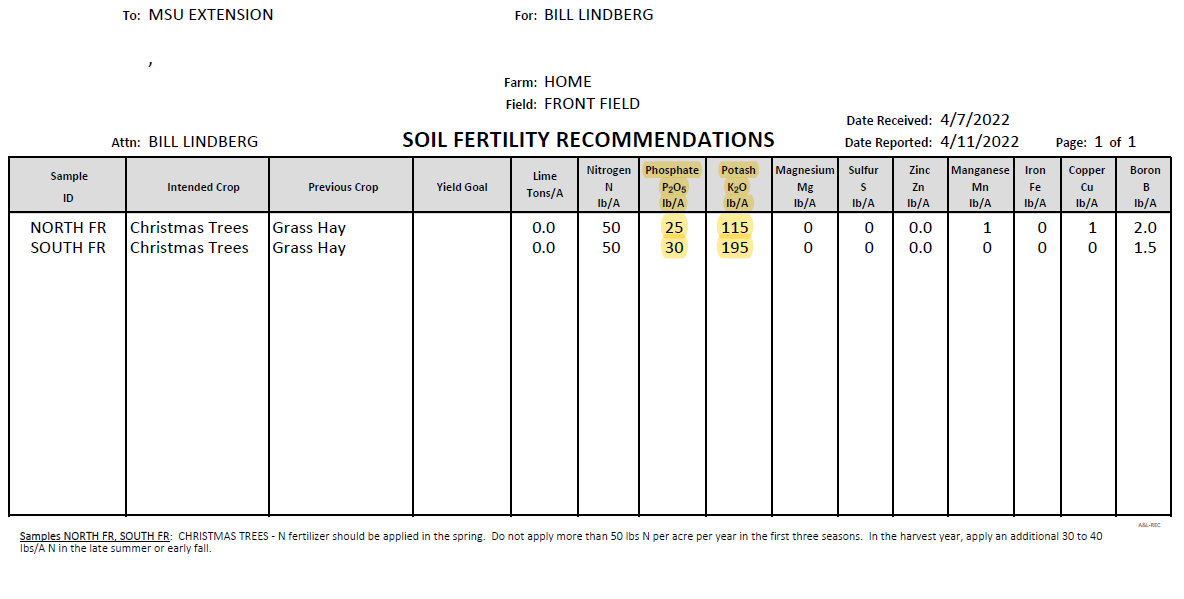
Common granular fertilizer products containing potassium include muriated potash (0-0-60) and sulfated potash (0-0-50). Muriated potash has a relatively high salt index (high potential for fertilizer burn) and is often applied in the fall when rains are usually more frequent. For in-rotation or Spring application, sulfated potash is the desired choice. Sulfated potash (0-0-50) applied at 230 pounds per acre will supply the necessary 115 pounds of K2O (potassium) for the north section, while 390 pounds per acre will supply 195 pounds of K2O recommended for the south section.
Micronutrients
In general, soils in Michigan typically have adequate amounts of micronutrients to meet the needs of Christmas trees. Manganese (Mn) and iron (Fe) are sometimes deficient in Christmas trees, but the issue is often related to soil pH that is above the target range for the species. Occasionally, a soil test may indicate a deficiency of boron (B), which can usually be alleviated with a low rate (1-2 pounds per acre) of boron (Photo 2).
Timing of application
For immobile elements (e.g., P) pre-plant fertilization and incorporation are best. For applications during the rotation, MSU Extension recommends fertilizing two weeks before bud-break. Fertilizing in early spring corresponds with the re-initiation of root growth (Photo 3), minimizes volatilization of urea as temperatures are usually cool, and often coincides with spring rains. Fertilizer applied after new vegetative growth starts may also cause damage of tender tissue if it sticks on new growth post application.

Potential issues with fertilization
Improper fertilizer applications can cause negative outcomes for growers. Applications made in a concentrated area near tree roots can cause plant injury or death (Photo 4). All fertilizers have a salt content and over application draw water away from roots. MSU Extension recommends applying a uniform fertilizer application around the drip lines of trees.

Lastly, both nitrogen and phosphorus can be environmental contaminants in surface and ground water. During rain events, nutrients can be moved by surface water flow from fields to waterways. This can lead to environmental issues including algal blooms and fish kills. Leaching through the soil profile is also a concern for nitrogen. Fields on lighter soils are more at risk and can result in contaminated aquifers. Applications made at the right time, rate and form are less likely to become Fan environmental issue.



 Print
Print Email
Email

