Spring rains can bring more than May flowers
Acid rain or acid deposition is a serious problem that affects all aspects of our ecosystem.
 Most people know the familiar phrase “April showers bring May flowers.” But also important to note is that April showers can bring with them acid rain. Acid rain refers to deposition from the atmosphere that contains high amounts of acid, primarily sulfuric and nitric. This deposition can be wet or dry. Wet deposition is precipitation that collects acids out of the atmosphere and carries them to the Earth’s surface. Dry deposition includes particles and gases from dust and smoke that stick to the ground and other surfaces. Dry deposition can later be washed into surface waters by new rain or snow fall.
Most people know the familiar phrase “April showers bring May flowers.” But also important to note is that April showers can bring with them acid rain. Acid rain refers to deposition from the atmosphere that contains high amounts of acid, primarily sulfuric and nitric. This deposition can be wet or dry. Wet deposition is precipitation that collects acids out of the atmosphere and carries them to the Earth’s surface. Dry deposition includes particles and gases from dust and smoke that stick to the ground and other surfaces. Dry deposition can later be washed into surface waters by new rain or snow fall.
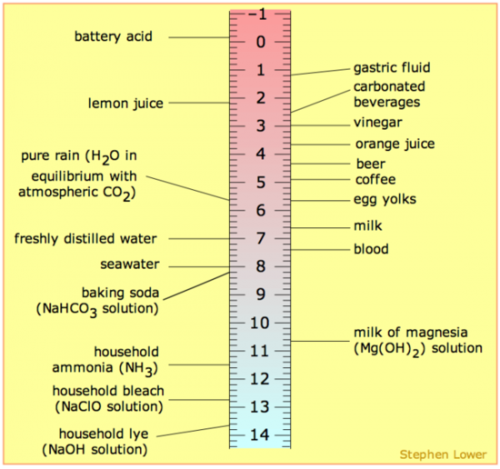
Diagram at right: This diagram of the pH Scale shows the acidity and alkalinity of common products and compares them to acid rain and its impacts.
Acid rain is more appropriately called “acid deposition” because deposition includes all forms of precipitation including snow or sleet.
Acid deposition formation comes from two sources. Natural sources include volcanic eruptions and decaying vegetation. Man-made sources primarily come from fossil fuel combustion emissions of sulfur dioxide (SO2) and nitrogen oxide (NOx). These byproducts are created when coal or gas is burned in power plants or vehicles. Acid deposition occurs when these gases react with water, oxygen and other chemicals in the atmosphere to form acidic compounds (Read related: Acid rain reductions means accepting trade-offs).
Approximately two-thirds of all SO2 and one-quarter of NOX are fossil fuels, coal, oil and natural gas, used to produce electricity. SO2 and NOX are released from the smokestacks and carried by the wind to neighboring communities, states or countries.
Acid deposition is measured using the pH scale. The pH scale is a measure of the amount of acid found in water or liquids. The scale ranges from zero to 14 with 7 being neutral. The lower the number the more acidic the substance. The higher the number, the more alkaline. Pure water has a neutral reading of 7. Normal rain water tends to be slightly acidic due to carbon dioxide that it collects from the atmosphere. Any precipitation below 5.3 is considered acidic. It’s also important to remember that the pH scale is logarithmic, meaning that the increase or decrease between two numbers is 10 times – not one time - the change. So from a 6 on the pH scale is actually 10 times more acidic than a 5.
Acid deposition has a number of impacts on both the natural and man-made environment. The aquatic environment is impacted the most because it receives both wet and dry deposition. Over time, the diluted acid accumulates to lower the overall pH of the water body. Acid deposition on clay soils (found in Southeast Michigan) can release aluminum and magnesium, which can further increase acid levels. Estimates are that 50,000 U.S. and Canadian lakes have a pH below 5.3.
Forests are negatively impacted by acid decomposition. Acid rain can cause trees to lose their leaves, damage tree bark and stunt tree growth. This damage makes the tree susceptible to diseases, insects and extreme weather conditions. Acid rain soaking into the soil can kill microorganisms essential to plant growth and disrupt soil nutrients.
In addition to damaging the natural ecosystem environment, acid deposition negatively impacts the man-made environment. Acid deposition falling on buildings or statues reacts with the stone’s material and may cause it to disintegrate and wash away. Many historic buildings and monuments are showing signs of this type of damage. Modern building, bridges, vehicles and pipes both above and below ground can be corroded if they come in contact with acid deposition.
For more information on acid rain, visit the Environmental Protection Agency’s website.



 Print
Print Email
Email

