Beyond resistance, why is horseweed a headache for managers?
Michigan State University researchers study the factors that affect horseweed’s remarkable ability to develop as a winter or summer annual weed.

According to the most recent surveys of U.S. and Canadian farmers from the Weed Science Society of America, horseweed is among the top five most troublesome weeds in winter cereal grains, fruits, nuts and soybeans. Horseweed, also known as marestail, produces large numbers of seeds spread by the wind, is drought resistant, and is capable of rapid growth and regrowth after cutting. It is found in both organic and conventional fields, particularly in no-till or reduced till systems, with many populations resistant to glyphosate and ALS-inhibiting herbicides.
Further complicating management, horseweed has a highly flexible life cycle. Seedlings emerge across a broad window of time, with peaks in both fall and spring. Plants that emerge in fall typically follow a winter annual life cycle, forming a low-to-the-ground rosette to survive winter, then growing upright and starting to flower in spring (Figure 1). Plants that emerge in spring typically follow a summer annual life cycle, skipping the rosette stage and immediately growing upright (Figure 1).
New findings from Michigan State University (MSU) scientists show that summer annual and winter annual horseweed are not genetically different. Instead, seeds adjust their life cycle in response to the external temperature and moisture conditions they experience, which affect their internal levels of certain hormones, or signaling molecules. Unfortunately, this means that a single seed can produce two generations of offspring per year.
Gaining a better understanding of the factors affecting horseweed’s life cycle from emergence to seed shed is important for improving management. The effectiveness of chemical and mechanical interventions depends not only on how old a plant is, but also its traits. Further, the timing of seed shed influences the emergence timing in the next season.
Management takeaways:
- Changes in the proportion of summer versus winter annual horseweed in a field from year to year are likely due to differences in management decisions that affect seed shed timing and germination conditions.
- Horseweed will emerge whenever moist, well-lit, above-freezing conditions occur. Cover crops, tillage or preemergence herbicides can be used to prevent these conditions, with fall management needed to prevent winter annual horseweed establishment and spring management needed to prevent summer annual horseweed establishment.
- Seeds shed from plants of either life cycle type and can develop as plants of either life cycle type in the next generation, so both summer and winter annual horseweed must be managed to control horseweed in just one season.
- Summer annual horseweed is likely to present a greater management challenge due to increased herbicide resistance.
- More frequent management interventions may be needed to control horseweed since its window of seasonal emergence is likely to expand in the coming decades.
- If gibberellic acid is applied to a field, it is likely to increase the proportion of horseweed seeds in the seedbank that develop as summer annuals, but field trials are needed.
Patterns of horseweed seasonality are highly variable
Horseweed seeds generally require sunlight and warm temperatures (at least 46-57 degrees Fahrenheit) to germinate, according to prior research in Canada and California. With warmer winters predicted in the Midwest, it is likely that the germination window for horseweed will expand. Further, in a recent experiment, horseweed seeds readily germinated at 39 F, reaching 40% germination in one to three weeks. This means that horseweed may be less temperature-limited than previously thought. Instead, emergence patterns are more likely to depend on management practices that affect available light at the soil surface.
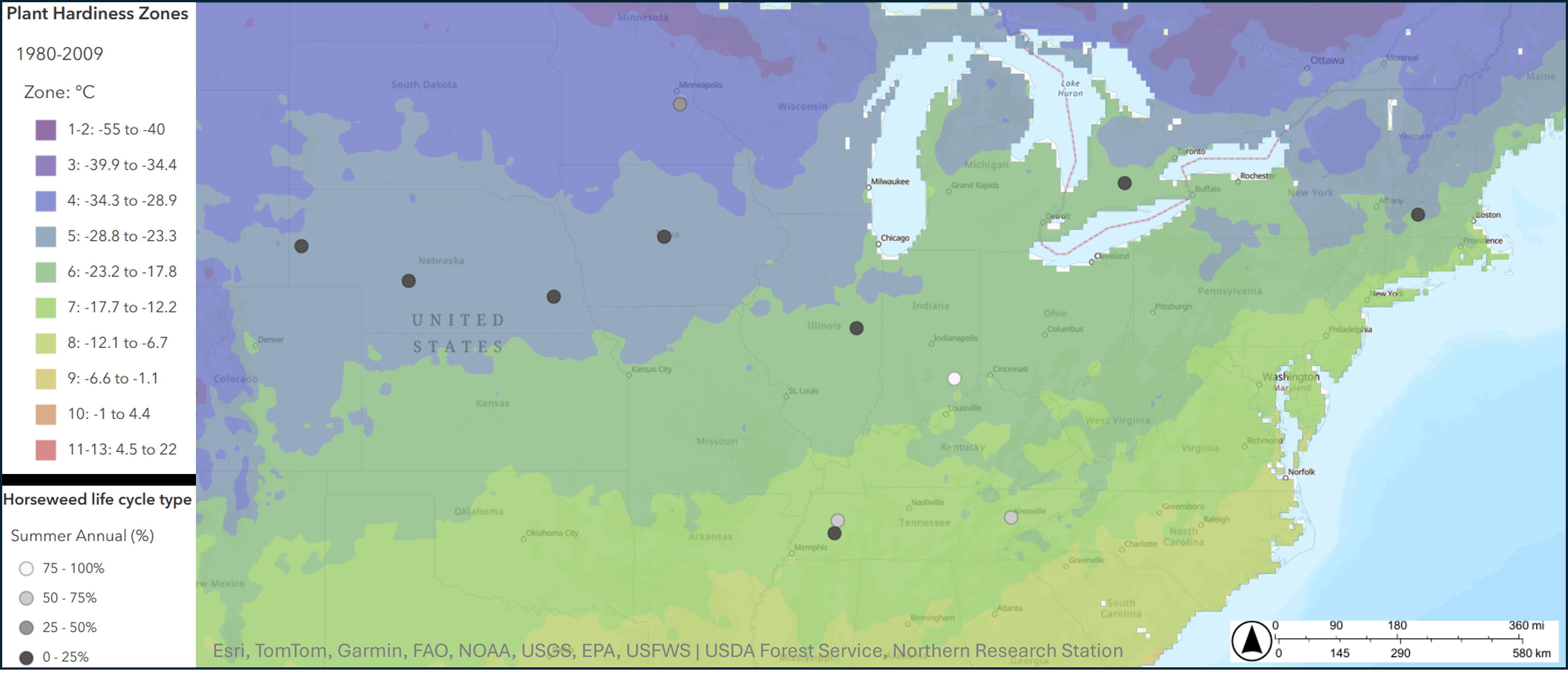
The flexible ability of horseweed to shift life cycles allows the plant to succeed in variable conditions. According to a study by Regehr and Bazzaz at the University of Illinois, with no management, fall-emerging horseweed plants produce more seeds. However, spring-emerging horseweed can do better in fields without early spring management. Preliminary field data suggest that seedlings transplanted into a field in fall that make an overwintering rosette perform better than those that do not, while seedlings transplanted into a field in spring that skip the rosette stage perform better than those that make a rosette. According to studies led by former MSU graduate students John Schramski and Justine Fisher, summer annual plants, and especially those from glyphosate-resistant populations, are more resistant to glyphosate partially because they do not retain as much of the chemical on their leaves.
Horseweed life cycle is primarily affected by seed temperature, not genetics
Both summer and winter annual horseweed are often observed in the same field. We now know from recent experiments in growth chambers that both life cycle types can produce seeds that develop into either life cycle type. This contrasts with cases like winter versus spring wheat, where seeds from winter wheat always develop into winter annuals, and vice versa. Instead of fields containing genetically distinct populations of winter and summer annual horseweed, seeds use environmental cues to adjust their development.
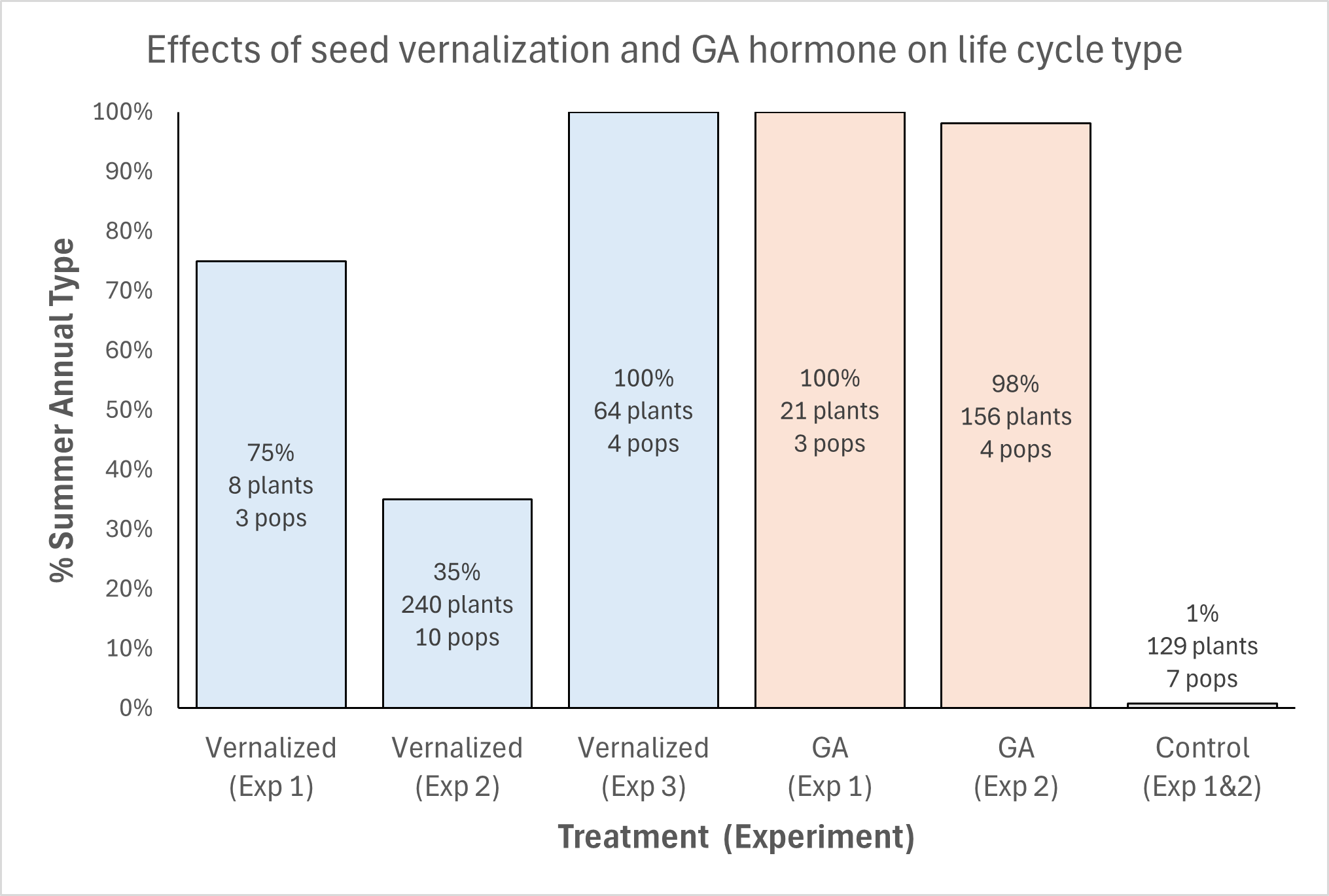
What environmental cues do these seeds rely on? To study this, MSU researchers collected horseweed seeds from four Michigan agricultural fields and germinated them in various environmental conditions. They then classified seedlings based on their growth traits into either winter annuals if they formed a rosette or summer annuals if they immediately grew upright. Only exposing seeds to cold and moist conditions (“seed vernalization”) affected life cycle type, with four weeks sufficient to cause all plants to develop as summer annuals (Figure 3).
Two follow-up experiments using additional Michigan seed sources found more variation in the response but confirmed a role for seed vernalization. In addition, the response to seed temperature was largely consistent within and between seed collection sites. This provides further confirmation that genetic differences between plants do not explain differences in life cycle.
These experiments also indicated that the mechanism for this response to cold temperatures is likely an increase in the production of the plant growth hormone gibberellic acid (Figure 3). This hormone is commonly applied to a variety of crops to promote uniform germination and speed up growth. Seed treatment or foliar spray with this hormone caused most horseweed plants to develop summer annual growth types.
Acknowledgements
I’d like to thank John Schramski, Eric Patterson and Christy Sprague for graciously sharing their data; student mentees Brooke Catlett, Ishwari Bhatt and Georgia Edmonds for assistance with the experiments; Kate Shaw and Mark Hammond at the Kellogg Biological Station for greenhouse and growth chamber management; and Second Spring Farm, Natural Cycles Farm and DeLano Farms for seed collection access.
This work was supported by the Education and Workforce Development Program of the U.S. Department of Agriculture’s National Institute of Food and Agriculture [2023-67011-40398 to RW] and the Research Experiences for Undergraduates Program of the U.S. National Science Foundation Division of Biological Infrastructure [2150104].



 Print
Print Email
Email


