Current Fungal Biology – The importance of the mycobiome in wound treatment
The human microbiome is defined as the assemblage of microorganisms that live in close association to the human body.
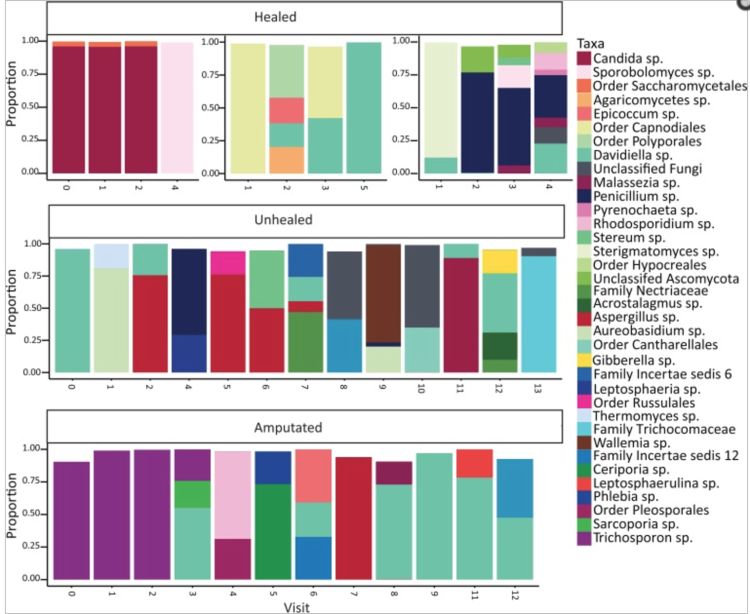
The human microbiome is defined as the assemblage of microorganisms that live in close association to the human body. These organisms may be mutualists, parasites, or simply just hanging on for the ride. However, how these populations grow, affect human physiology, and how they are structured is not well known. The human mycobiome, a subset of the human microbiome that includes only the fungal species of the microbiome, is even less well studied. Certain species that are a part of the human mycobiome, and indeed the human microbiome as well, are opportunistic pathogens that can cause systemic infections at wound sites if not properly treated especially in people with certain diseases or conditions, such as immunocompromised individuals or those with diabetes. A recent study, published in mBoi, into the effect of the human mycobiome on healing times of patients with diabetic foot ulcers (DFUs) found some interesting results.
Most importantly, they found that while the fungal community is not stable over time (Figure 1), and proportions of certain groups of fungi can predict healing times. They also found that even within the small spatial distance of the human foot, there were significant differences in the fungal communities present around DFUs based on the position on the foot (forefoot, midfoot, hindfoot) based on the Shannon Diversity index with the forefoot containing the highest diversity of fungal species with a gradient of decreasing diversity toward the hindfoot. This result has implications across a wide-range of medical paradigms; the human body contains a wide variety of different niches that may be colonized by a wide assemblage of fungal (and indeed bacterial) species, but more research needs to be done, both within and across different human subjects, to fully describe the colonization of these niches.
Unsurprisingly, they found that many of their most abundant Ascomycota species (10 out of 17) are ubiquitous in the environment. Species such as Candida albicans are some of the most commonly identified Ascomycota yeasts in the human mycobiome. On the other hand, some of the yeast species identified (namely, the Basidiomycota yeast Trichospora and Rhodosporium spp.) are capable of forming biofilms with bacteria that may complicate wound treatment and increase healing time in some patients. A collection of these biofilm forming yeasts were more commonly associated with decreased oxygenation of the wound and, as a result, less favorable outcomes like slow healing or amputation.
To read the article for yourself follow the link here.
Literature Cited:
- Kalan L, Loesche M, Hodkinson BP, Heilmann K, Ruthel G, Gardner SE, Grice EA. 2016. Redefining the chronic-wound microbiome: fungal communities are prevalent, dynamic, and associated with delayed healing. mBio 7(5):e01058-16. doi:10.1128/mBio.01058-16.



 Print
Print Email
Email



