Different types of limestone to increase substrate pH
Part 1 of a series on adjusting substrate pH will discuss how the type, particle size and hardness of limestone will effect substrate pH.

Michigan State University Extension provided recommendations on how growers can determine greenhouse substrate pH and reviewed corrective procedures on how to increase low substrate pH in a previous article, “Testing and corrective procedures for low substrate pH.” Many growers are testing incoming substrate pH and are reporting the pH of some batches of peat mixes are inconsistently low: between 4.0 and 5.4. The recommendations on correcting substrate pH will differ significantly depending on the pH of the incoming substrate.
Growers should always check the initial substrate pH of each bale before amending any pulverized 100- or 200-mesh (particle size) calcitic or dolomitic limestone. The initial substrate pH will be the foundation and will help determine how many pH units increase are needed. Therefore, a grower with a bale of peat:perlite mix with a pH of 4.0 will need to amend the mix with more lime to raise the pH to 6.0 than if a bale had a pH of 5.0.
In this first article of a two-part series, MSU Extension will provide an overview of the types of lime that can be used to adjust pH and factors that effects its reactivity in the substrate.
Two types of limestone are commonly available and used in adjusting the pH of greenhouse and nursery substrates: calcitic limestone and dolomitic limestone (see table). Hydrated limestone [Ca(OH)2] is another option, but it is less frequently used because it is more caustic.
|
Two common types of limestone used to adjust pH in greenhouse and nursery substrates. | |||
|---|---|---|---|
|
Types of limestone |
Active ingredient |
Molecular formula |
Notes |
|
Calcitic limestone |
calcium carbonate |
CaCO3 |
|
|
Dolomitic limestone |
calcium magnesium carbonate |
CaMg(CO3)2 |
|
Horticultural limestones are also available in two common particle size distributions, pulverized (100 mesh; 60 percent passing through a 100 mesh screen, see Photo 1) or superfine (200 mesh; 60 percent passing through a 200 mesh screen, see Photo 2). These terms are often used interchangeably in the horticulture industry to describe the particle size or mesh size distributions.

Photo 2. Superfine or 200 mesh calcitic limestone is the most reactive in adjusting substrate pH because the particle are smaller, similar to powder sugar.
In general, superfine (200 mesh; smaller particles) is more reactive in adjusting substrate pH compared to pulverized (100 mesh; larger particles) because superfine limestone has more surface area and has a greater cation exchange capacity (CEC) (Photo 3). Growers need to be aware that the pulverized limestone, which is less reactive, may slowly increase the pH and then slowly decline once it has all broken down. Similarly, superfine limestone will quickly change the pH of the substrate, but the pH could then quickly decrease over the course of a few weeks. Therefore, soilless substrate manufacturers often mix both particle sizes into their mixes, which helps to regulate the pH more consistently throughout the crop’s growing season.
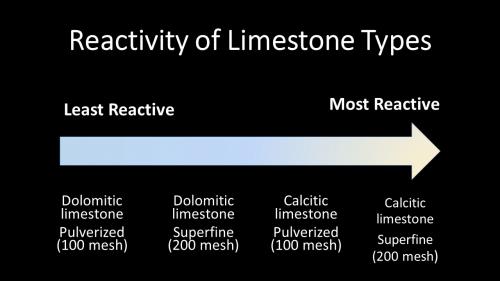
Photo 3. Pulverized dolomitic limestone is the least reactive while superfine calcitic limestone is the most reactive in the substrate.
Limestone hardness refers to the soft or hard and smooth mineral structure of limestone crystals. Agricultural and horticultural limestones are considered soft crystals, reacting quickly with acid, which are more desirable in adjusting the pH of substrates formulated with peat moss, pine bark or coconut coir. Therefore, determining the rate of limestone incorporation required to adjust substrate pH depends on the components of the substrate and the type, particle size and hardness of limestone incorporated.
Part 2 of this series will discuss the commonly-used limestone products for adjusting soilless substrate pH.
Other articles in series
Thank you to Drs. Roberto Lopez and Brian Whipker for their reviews.



 Print
Print Email
Email




