Calcium Carbonate Clogging
For the pdf version of this bulletin, click here.
 3.4. Calcium carbonate clogging
3.4. Calcium carbonate clogging
Calcium carbonate clogging of drain pipes can occur in soil with high pH and dissolved Ca+2 concentration at high temperatures.
A calcareous soil has high calcium carbonate, which increases dissolved Ca+2 concentration in water. As the calcium-rich water enters the pipe, pressure drops because water moves from a saturated soil into a partially open pipe. The pressure drop releases carbon dioxide (CO2) gas from the water, thereby increasing the pH of water. At the same time, high temperatures during the summer decrease the solubility of calcium carbonate in water, which leads to the formation of an insoluble calcium carbonate.
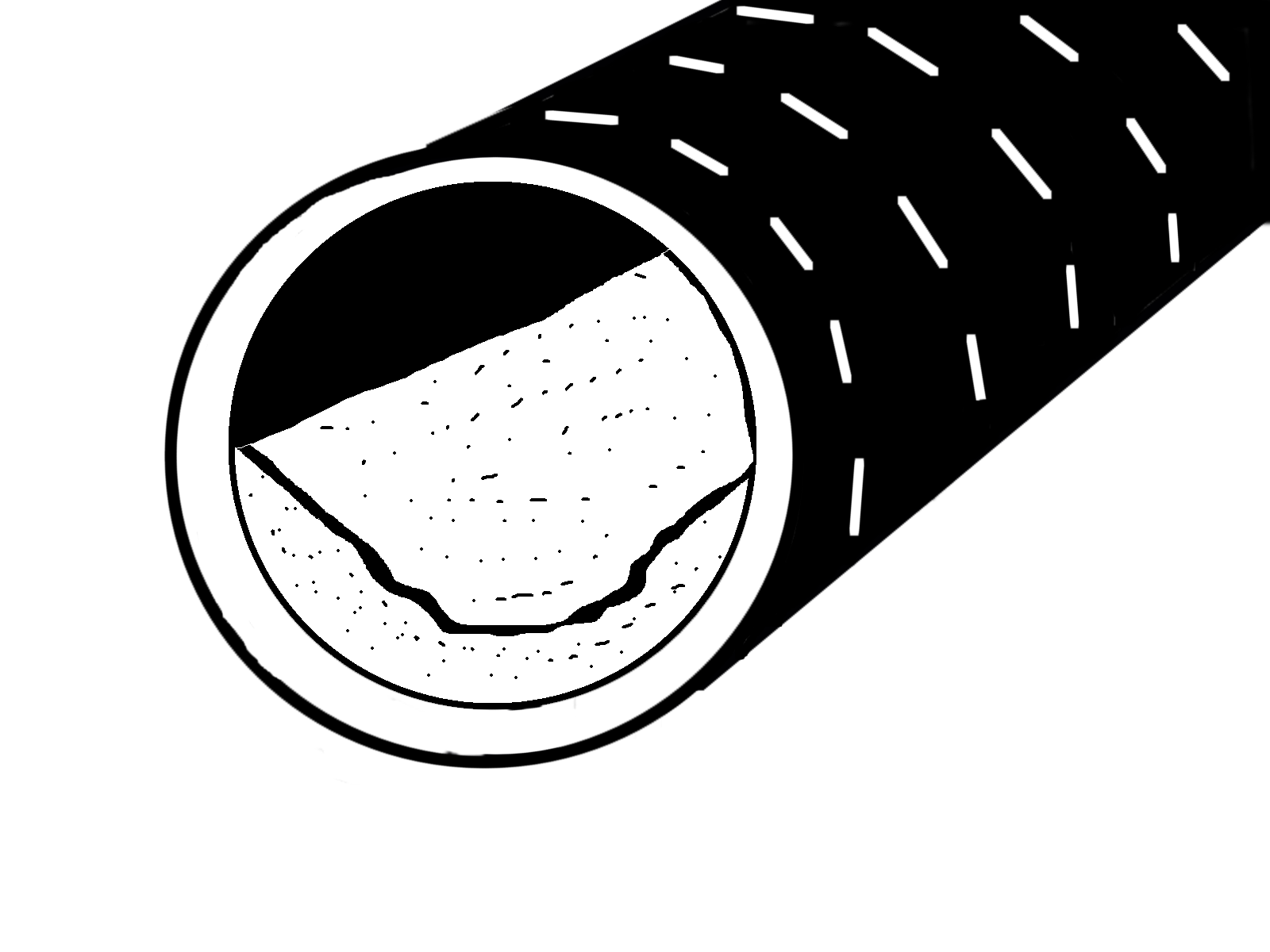
Calcium carbonate can cause perforation clogging, or in rare cases, blockage of the pipe. Figure 5 shows a sample of a hard-pan layer of calcium carbonate that deposited on the bottom of a 12-inch main pipe in southwest Minnesota. That location was a few miles from a calcareous fen. Calcareous fens are wetlands with a steady supply of calcium-rich groundwater, which provide one of the ingredients for calcium carbonate clogging.
Calcium carbonate clogging has been observed in southwest Minnesota mostly in drain pipes with a steep grade. A pipe with a steep grade (without a breather) can increase the chance of calcium carbonate clogging. This is because the pressure drop is greater when water enters a pipe with a steeper grade (without a breather) than a flatter grade.



 Print
Print Email
Email