Nitrogen Management in Christmas Trees
DOWNLOADJanuary 31, 2019 - Questions about this information? Contact us.
Application of commercial fertilizer has become an essential part of plantation management for many Christmas tree producers. Soil testing, foliar analyses and observations of tree growth and response are all used in establishing the need for fertilization in Christmas tree plantations. The potential exists to improve the growth and quality of many Christmas tree species through fertilization. However, for fertilization to be most effective, other environmental factors affecting tree growth such as the right site for the species, soil moisture considerations and effective weed control need to be in place.
Nitrogen
Nitrogen is a major structural component of many organic compounds found in plants including chlorophyll, proteins, nucleic acids and enzymes. Proper nitrogen nutrition is commonly associated with development of aboveground parts of the tree, including branch length and diameter growth, bud number and size, and foliage density and color. The most common visible symptom of nitrogen deficiency in Christmas trees is yellowing and stunting of needles. Because nitrogen is a mobile nutrient within the tree, older needles yellow first as nitrogen can move to the younger foliage.
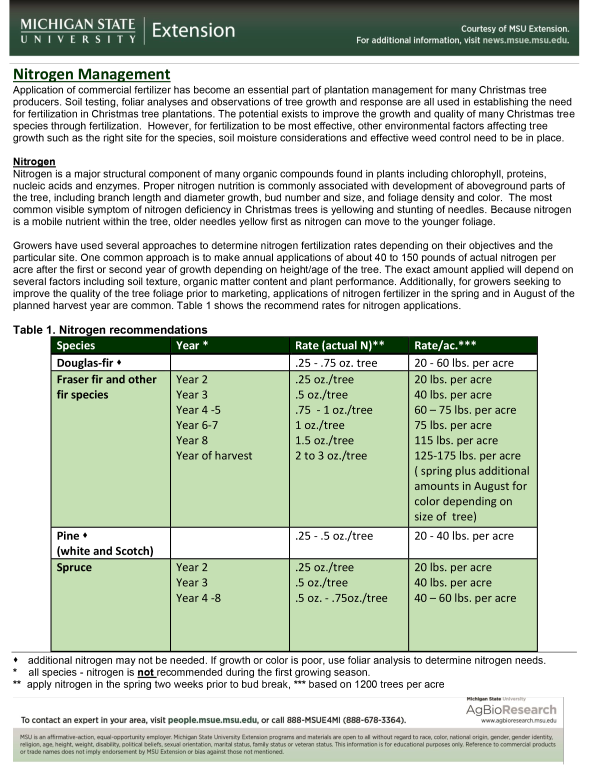
Growers have used several approaches to determine nitrogen fertilization rates depending on their objectives and the particular site. One common approach is to make annual applications of about 40 to 150 pounds of actual nitrogen per acre after the first or second year of growth depending on height/age of the tree. The exact amount applied will depend on several factors including soil texture, organic matter content and plant performance. Additionally, for growers seeking to improve the quality of the tree foliage prior to marketing, applications of nitrogen fertilizer in the spring and in August of the planned harvest year are common. Table 1 shows the recommend rates for nitrogen applications.
| Table 1. Nitrogen recommendations | |||
|---|---|---|---|
| Species | Year* | Rate (actual N)** | Rate/ac.*** |
| Douglas-fir | .25 - .75 oz. tree | 20 - 60 lbs. per acre | |
| Fraser fir and other fir species |
Year 2 Year 3 Year 4 -5 Year 6-7 Year 8 Year of harvest |
.25 oz./tree .5 oz./tree .75 - 1 oz./tree 1 oz./tree 1.5 oz./tree 2 to 3 oz./tree |
.25 oz./tree .5 oz./tree .75 - 1 oz./tree 1 oz./tree 1.5 oz./tree 2 to 3 oz./tree |
| Pine (white and Scots) | .25 - .5 oz./tree | 20 - 40 lbs. per acre | |
| Spruce | Year 2 Year 3 Year 4 -8 |
.25 oz./tree .5 oz./tree .5 oz. - .75oz./tree |
20 lbs. per acre 40 lbs. per acre 40 – 60 lbs. per acre |
* all species - nitrogen is not recommended during the first growing season.
** apply nitrogen in the spring two weeks prior to bud break.
*** based on 1200 trees per acre.
Fertilizer Materials
Trees absorb nitrogen from the soil in in two forms, ammonium (NH4+) and nitrate (NO3 ˉ). Nitrogen fertilizers for use on Christmas trees usually include urea or ammonium. Conifers may respond poorly if nitrate is the only source of nitrogen. Ammonium can be held on the soil colloids, and will resist leaching while nitrate ion will be will be subject to rapid leaching.
Common fertilizer materials used as nitrogen sources in Christmas tree production include;
Ammonium sulfate (21-0-0-21) is a strongly acid-forming nitrogen fertilizer that is relatively easy to handle and provides sulfur. It is often used by growers on sites where the pH is higher than desired, or where sulfur is deficient. On some sites providing about 5 percent of the total nitrogen fertilizer in this form may deepen and enrich green color.
Diammonium phosphate (16-46-0), commonly referred to as DAP is somewhat expensive as a nitrogen source,
especially when adequate phosphorus is available. In most instances, it is used when both nitrogen and phosphorus are required.
Urea (46-0-0) is another commonly used, inexpensive nitrogen fertilizer. At 46 percent nitrogen, has the highest nitrogen content of any solid fertilizer. In the soil, urea rapidly reacts with water to produce ammonium ion. If urea is applied on bare soil, during warm weather, as much as 30 percent of its nitrogen content can be lost through volatilization as ammonia. This can minimized by a light incorporation or application when there is a high probability of rain within 24 to 48 hours. Care should be exercised when using urea to avoid "burning".
Other nitrogen materials such as slow release forms of urea and calcium nitrate are available but are not typically used due to their higher cost.
Conversions
Nitrogen recommendations are made in ounces per tree or pounds per acre of actual nitrogen. To convert these
recommendations to ounces or pounds of fertilizer material the following formula can be used;
Ounces or pounds of actual nitrogen = Ounces or pounds of material
Percent of nitrogen/100
Example:
2 ounces of actual nitrogen per tree = 4.35 ounces of urea per tree
0.46 (urea 46-0-0)
| 1 ounce of nitrogen per tree using various nitrogen sources | Ounces per tree |
|---|---|
| Urea (46-0-0) | 2.2 |
| Ammonium sulfate (21-0-0) | 4.75 |
| DAP (16-46-0) | 6.25 |
Adapted from:
J. Darling, D. Rothstein, “Estimating Crop Nutrient Demand in Fraser Fir”, Great Lakes Christmas Tree Journal, (Summer 2008):4-8
M. R. Koelling, Randall B. Heiligmann, “Soils and Christmas Tree Production Part 10, Fertilization Recommendations,” Michigan Christmas Tree Journal, (Spring 1997):27-32
M. R. Koelling, Randall B. Heiligmann, “Soils and Christmas Tree Production Part 11, Fertilizer Materials,” Michigan Christmas Tree Journal, (Summer 1997): 8-13,42



 Print
Print Email
Email