When considering how best to prevent disease spread on-farm, what is good for the goose is good for the gander (and the pigs)
Control at the source and further prevention is critical to preventing disease spread both on the farm and in humans.
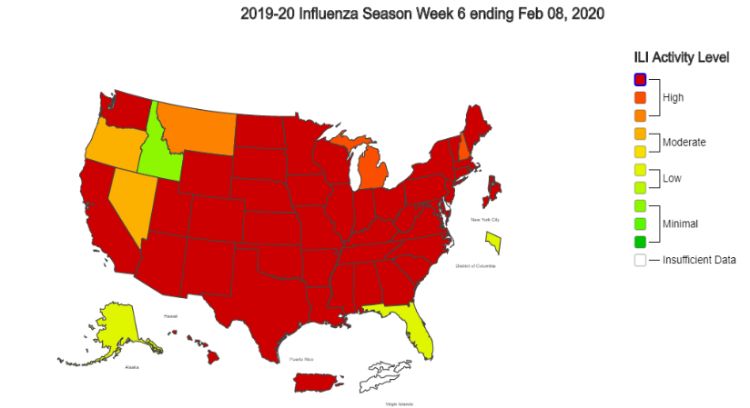
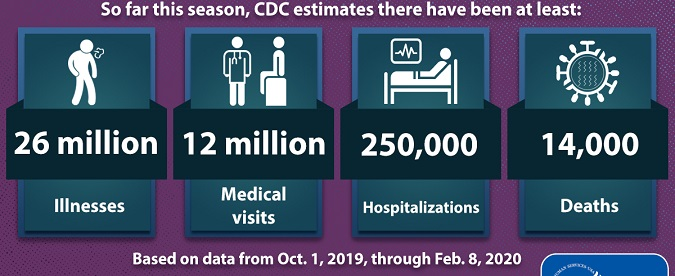
Reviewing surveillance reports detailing disease outbreaks can be nerve-wracking. If you have young children, ageing parents, or have a weakened immune system, the ‘cold and flu season’ can be potentially devastating. In an attempt to inform the public and implement activity aimed at preventing further spread of disease, the US Centers for Disease Control and Prevention (CDC) routinely publishes reports indicating current viral activity, including geographic spread trends (as shown in the map above) and characteristics of seasonal outbreaks (e.g. numbers of individuals with reported disease, number of hospitalizations, number of deaths, etc.).
The end of 2019 also brought with it the emergence of a novel (new strain of Coronavirus (COVID-19), with the first reports of person-to-person spread in the United States occurring on January 30, 2020. Almost immediately, the US Health and Human Services Secretary, Alex Azar II, declared a Public Health Emergency (PHE) on January 31, 2020 to allow for the immediate mobilization and response of the US healthcare system
to rapidly identify and treat those affected and to minimize further spread.
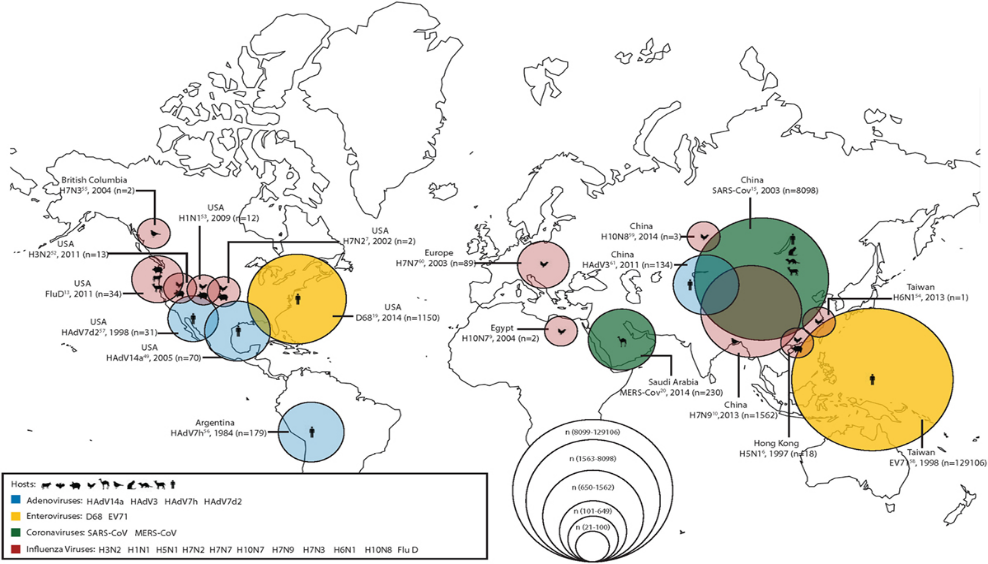
With the identification of (novel) pathogenic organisms (e.g. viruses and bacteria), risk assessments are quickly performed to determine the source (‘ground zero’) and characteristics allowing for spread of disease (e.g. mode of transmission, requirements for replication), including whether there is a potential for disease to be transmitted between humans and animals (zoonosis). In a recently published paper, Bailey and others highlight the threat for viral spread with a focus on the ‘human-animal interface’ and four respiratory viruses as shown in the map below. What becomes readily obvious is the influence of host species interaction in the prediction of emerging disease potentially resulting in an epidemic (actively spreading disease) or even pandemic (geographic spread, affecting an entire country or the world). The same paper demonstrates the need for continued and perhaps more intensive cross-functional and cross-species surveillance activity in order to more rapidly identify and effectively contain and prevent spread of disease.
The prevention of transmission of seasonal influenza in humans routinely includes vaccination, the recommendation that sick individuals stay home (isolation) and the encouragement of cough etiquette and hand hygiene. Work in a healthcare setting or caring for a sick individual may warrant the implementation of ‘droplet precautions’ (the use of appropriately fitting and filtering respirators (masks)). Current efforts to prevent/minimize the spread of COVID-19 include the identification, isolation and treatment of known affected individuals, identification and isolation (quarantine) of those potentially exposed and limiting/restricting movement of humans and goods by air, boat or rail to minimize spread to other countries.
Obvious parallels can be drawn between humans and food animals with respects to the identification and control of seasonal and emerging disease. In a recent publication, Beltran-Alcrudo and others review transboundary swine diseases (TSDs) of major concern including African Swine Fever (ASF), Classical Swine Fever (CSF), Porcine Reproductive and Respiratory Syndrome Virus (PRRSv), Foot and Mouth Disease (FMD), and the recent increase in incidence (new cases) of Porcine Epidemic Diarrhea Virus (PEDv). The incidence and prevalence (existing cases) of disease is ‘dynamic’ in both time and space, with disease waning and then reemerging. Seasonal shifts in disease incidence and prevalence domestically may be found on the swine health information center website. Surveillance data undercounts new cases of disease because cases from herds, which have become free of disease and then become re-infected (e.g. with PEDv or Porcine Delta Coronavirus (PDCOv)), are not included in the counts. Similar reports have indicated that Porcine Reproductive and Respiratory Syndrome Virus (PRRSv) re-infection of wild type strains into pig herds are both devastating and persistent despite eradication programs. This has resulted in a persistent (endemic) status in North America.
In anticipation of ASF, teams comprised of staffs from regulatory agencies, veterinarians and producers have collaborated to develop phased protocols to address any future outbreak. As with COVID-19, plans are in place to limit or altogether halt transportation (enforced quarantine) should ASF be identified in North America, effectively limiting disease spread. Programs are in place to identify and destroy sources of pathogen in commercial goods (e.g. feedstuffs). Depopulation plans specify handling of mass mortality and adequate disinfection including sufficient latency for reintroduction of naïve animals to reduce the likelihood of infection of the new herd.
It is well known that the most efficient and successful means to control exposures to virus, or bacterium and risk of disease, irrespective of whether the focus is human or animal, is to enact control at the ‘source’, or in other words, actively work to prevent pathogen transport by the host organism. This requires quarantine and isolation, targeted and appropriate hygiene practices and ‘droplet precautions’ to control spread of both emerging (COVID-19) and seasonal (Influenza) disease in humans. Implementation of appropriately modified practices are also effective in the control of pathogens affecting food animals. Unfortunately, routine management practices performed at livestock facilities – even when in compliance with established biosecurity protocols - have the potential to disperse (transport) pathogens to naive pigs on-site or at distant facilities or re-infect existing herds (e.g. PEDv and PRRSv).
When developing your biosecurity plan, including Perimeter Buffer Zone (PBA) and Line of Separation (LOS), consider and identify all activities and ‘touch points’ where a host (human or animal), fomite (inanimate object or material that can carry pathogen) or process (activity) has the potential to physically transport or disperse (e.g. in mists/droplets or dusts) pathogen. This risk assessment should also include the behavior (approach) by which tasks are completed. Items to consider should include, but not be limited to, the following:
- Use of ‘common’ tools (e.g. hoses, pitchforks, sorting boards, wheelbarrows, various cleaning implements (e.g. brooms) and equipment (e.g. tractors) that have the potential to be in contact with sick animals or animal fluids and/or excrement and healthy animals/clean areas.
- Air movement within the facility (forced ventilation vs natural ventilation, use and placement of fans). Is there a potential for respiratory secretions or contaminated dusts to become airborne and move from isolation areas to pens with healthy pigs?
- On-farm vehicle and pedestrian traffic. Is it possible for vehicles (cars, tractors, gators) and foot traffic – activity not already included in biosecurity protocols – to contact potentially contaminated sources (e.g. puddles, dirt/dusts) and track them into housing areas containing naïve pigs?
- Manure handling and pumpdown. At any point, is it possible for aerosols from contaminated slurry in pits to become aerosolized and dispersed into barns? Is there sufficient distance between the slurry in pits and floor of the pens? Are pigs able to inhale contaminated aerosols (or even foam) when fans in pits are turned on? Are aerosols generated during pumpdown? If yes, do they have the potential to disperse and be transported via air movement back into the barns (re-infecting herds)? Is there splashing and puddling on roadways such that vehicle or pedestrian traffic has the potential to transport pathogen to distant areas on the property?
- Mortality management. Are implements used to move animals sufficiently disinfected (including tractors) prior to coming in contact with ‘clean’ material? If composting, when forming or turning piles, is care taken to minimize airborne dispersion of potentially contaminated material that has not yet reached temperatures sufficient to inactivate pathogen?
- Zoonotic transfer. Are there provisions in place to prevent sick employees from coming in contact with pigs (e.g. administrative controls in the case of influenza or other viruses)? Is personal protective equipment (PPE) readily available to protect employees from coming into contact with sick pigs? Has training been performed such that employees effectively don and doff PPE in such a way so as to minimize the potential for contamination of their regular clothing?
Results of a risk assessment will allow for the development of cost effective and often readily implementable strategies (procedures) to control movement of pathogen. Examples of methods used to control dispersion are generally site/process specific, but may include interventions such as the use of ‘wet methods’ to minimize dispersion of dusts containing pathogen or slowing down the fill-rate when pumping out manure pits or lagoons to minimize aerosolization, splashing and puddling in roadways. The regular use of disinfectants on tools/farm implements and assuring sufficient contact time to kill pathogen is another easy-to-implement means to control pathogen spread.
Implementing ‘source controls’ allow for the potential to not only mitigate endemic disease within herds, but also protect against the transmission of seasonal and emerging disease. For assistance in conducting a site/process-based risk assessment or identifying appropriate source control strategies, please contact your MSU Extension team:
- Melissa Millerick-May milleric@msu.edu.
- Madonna Benjamin gemus@msu.edu
- Casey Zangaro zangaro@msu.edu
- Elizabeth Ferry franzeli@msu.edu
- Dale Rozeboom rozeboom@msu.edu
- Dave Thompson thom1637@msu.edu



 Print
Print Email
Email